
Jun 26, 2017
Five years ago, I “graduated” from a 37-year career as an orthopedic surgeon with a practice based on hip and knee replacement surgery for arthritic joints. Trading my scalpel for a needle, I entered the new discipline of Cellular Orthopedics with a goal of helping patients delay, at times avoid a joint replacement for the arthritic hip and knee joint. In my transition, I introduced the same integration of research, patient care, and education into Regenerative Medicine that I had pioneered as the head of a joint replacement program at a major-medical center in Chicago, helping that center emerge as one of the five most recognized programs in The United States.
At the beginning and continuing to this day, Cellular Orthopedics has been based on the use of a patient’s own Platelet Rich Plasma and Bone Marrow Concentrate as the source for providing pain relief and functional improvement to a patient experiencing impairment from an arthritic joint. I have almost five years of outcomes data to support my therapeutic recommendations. Platelet Rich Plasma offers healing and pain relieving promise for the patient; while the Stem Cells and Growth Factors in Bone Marrow Concentrate offer regenerative potential in addition, all this while FDA compliant.
Over the past year however, there has been an explosion in the marketing of untested and unproven alternatives continually introduced under the Regenerative Medicine umbrella. Web sites make unsubstantiated claims, purchased media campaigns become a source of false advertising and courses both live and on-line promise expertise credentialing in 48 hours.
As a response to this chaos, I have founded The Center for Orthobiologic Clinical Trials whereby I am able to validate or challenge the plethora of claims and assist my patients in decision making based on scientific evidence; while at the same time, contributing to the emerging discipline of Interventional Orthopedics. I now have gathered the largest Data Base with long term follow-up of which I am aware documenting the Outcomes of Bone Marrow Concentrate, Amniotic Fluid Concentrate and mechanically emulsified, Adipose Derived, Lipogems.
The next step is to study the outcomes of combining cellular orthopedic interventions into the joint with the added step of injecting Bone Marrow Concentrate and Platelet Rich Plasma into the bone adjacent to the joint. I am currently recruiting patients for the latter trial. For those who meet inclusion criteria, there is a discount for said care. Interested patients should call (312) 475-1893 on Monday or Thursday.
If you want to become better informed, browse my website www.sheinkopmd.com.
You may watch my webinar at www.ilcellulartherapy.com or call central schedulaing at (312) 475-1893 for a new patient consultation.
Tags: Amniotic Fluid Concentrate, Bone Marrow Concentrate, cellular treatment, Hip pain, joint replacement, knee pain, lipogems, mechanically emulsified Adipose Derived, Mesenchymal Stem Cell, Osteoarthritis, PRP, regenerative medicine
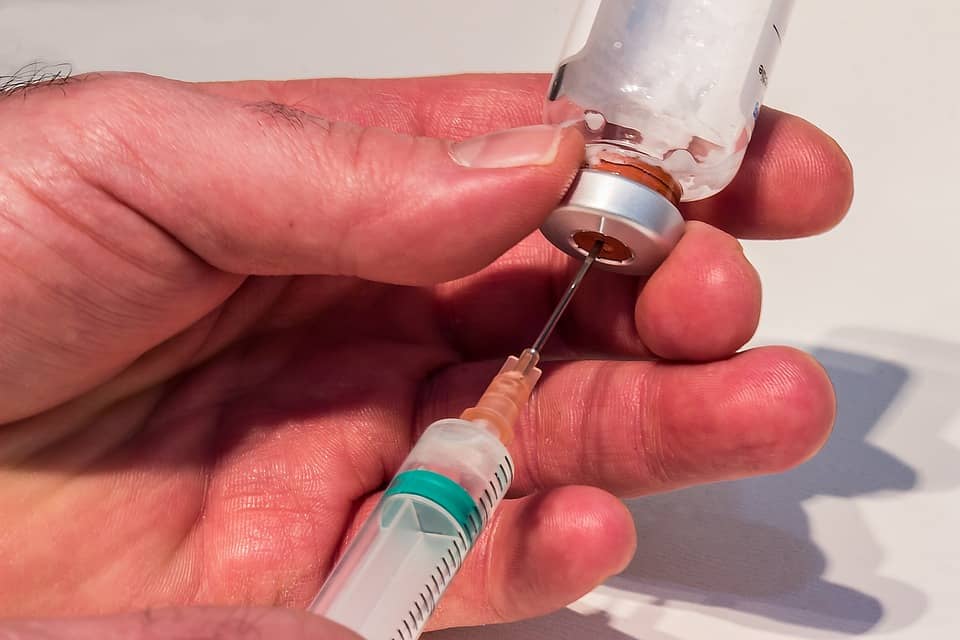
Jun 12, 2017
The lack of scientific foundation in stem cell marketing is all around us and negatively impacting those doing the right thing in the evolving discipline of Regenerative Medicine. Yesterday afternoon, a patient for whom I successfully completed a Bone Marrow Concentrate/Stem cell procedure presented to the office for a follow-up visit. She was accompanied by her husband who was experiencing progressive limitation attributable to an arthritic left knee. Because of my patient’s successful experience, her husband had determined now it was his turn. After the intake, I provided the customary explanation of what was to take place. During the question and answer follow-up, both husband, the new patient, and wife, the successful outcome, wanted to know why hers had worked whereas several of their friends had not enjoyed successful outcomes after amniotic fluid interventions.
The explanation is straightforward and based on a precedent, the fact speaks for itself. While Bone Marrow is full of Adult Mesenchymal Stem Cells and Growth Factors when harvested, processed, concentrated and reinjected into the symptomatic joint within 60 to 90 minutes after the harvesting; Amniotic Fluid has no living stem cells after sterilizing, freezing and fast thawing. Restated, Amniotic Fluid has little if any regenerative potential. Why am I able to make said statements in the face of such aggressive marketing claims regarding amniotic fluid? In addition to my work clinically and scientifically with Bone Marrow Derived stem cells and growth factors, I am the Principal Investigator in a clinical trial wherein amniotic fluid both frozen and fast thawed, and most recently, Lyophilized, has been used in lieu of hyaluronic acid to reduce or possible relieve the symptoms of osteoarthritis for six to 12 months. At no time did the largest amniotic product based pharmaceutical company in the United States suggest there are viable stem cells in amniotic fluid nor did they make any claim for regenerative potential. Returning to my office encounter, during our continued discussion, I learned that those who had opted for the amniotic fluid injection had paid more for the injections than I charge for the Bone Marrow intervention. So, think about the harm done to the “victims” as well as the public in general. The trusting patients paid for a regenerative procedure that they never received. The patients believing that the stem cell procedure didn’t work are now considering total joint replacements.
How might you protect yourself if you are considering a means by which you might postpone or avoid a joint replacement for arthritis? Make sure you choose a residency and fellowship trained interventional specialist. Second, ask the clinician to share his or her scientific outcomes data.
If you want to become better informed, browse my website www.sheinkopmd.com.
You may watch my webinar at www.ilcellulartherapy.com or call to schedule a consultation (312) 475-1893.
Tags: ACL Injury, arthritis, Bone Marrow Concentrate, Clinical Trial. Mitchell B. Sheinkop, Hip, Interventional Orthopedics, joint replacement, Knee Pain Relief, Mesenchymal Stem Cell, Orthopedic Surgeon, regenerative medicine, stem cell treatment

Jun 1, 2017
It is now over four years since I began the most comprehensive outcomes clinical trial ever undertaken in which Bone Marrow Concentrate was used to reduce pain, improve function, increase activities and alter the progression of osteoarthritis in a knee joint. At the start, it was generally believed that the adult mesenchymal stem cells would reproduce themselves and emerge as cartilage therein regenerating the joint. Continued scientific investigation has taught us that the adult mesenchymal stem cell acts as the conductor of a complex bio-immune process in conjunction with growth factors. One such growth factor is an endogenous polypeptide molecule, Transforming Growth Factor -Beta (TGF-ẞ). There are many other growth factors derived from the Bone Marrow Concentrate that play a role but that discussion is beyond the scope of this Blog. Additionally, be aware that Platelet-derived growth factor attracts mesenchymal stem cells and can stimulate proteoglycan production and chondrocyte proliferation. Incidentally, should you decide to seek consultation with one of the plethora of so called regenerative medical specialists populating the internet and advertising in the media, before you go, print my blog and ask them relevant questions pertaining to the science. You make be surprised to learn you as a potential regenerative consumer know more about the subject than the highly visible marketing provider.
Getting back to the clinical trial, the recruiting process of 50 patients ended two years ago and now we have two to four year of outcomes data to statistically analyze; that scientific process will be completed next week and presented at the Orthobiologic Institute meeting taking place in Las Vegas, June 8 to 10. For the first time, real outcomes data having been analyzed using the same criteria I used in my 37- year career as a joint replacement surgeon and head of a joint replacement program at a major medical center in Chicago will be presented to the regenerative medicine community. Unfortunately, I am unable to control the charlatans and camp followers who will attend the meeting and even try to use my data for their marketing. I choose to share my data as a challenge to those who seek to market and advertise stem cells for every malady known to mankind.
If you want to become better informed, you may access my website www.sheinkopmd.com.
You may watch my webinar www.ilcellulartherapy.com or call to schedule a consultation 312 475 1893.
Tags: bone marrow, Clinical Trial. Mitchell B. Sheinkop, Concentrated Stem Cell Plasma, Growth Factors, Mesenchymal Stem Cell, proteoglycan, stem cells, Transforming Growth Factor -Beta

May 18, 2017
Also known as degenerative joint disease, osteoarthritis is the most common joint disorder, and continues to be the leading cause of impaired quality of life in the United Sates. While OA is defined as the progressive loss of cartilage structure and function; that definition has most recently been expanded to include changes to bone, tissues within and around the joint and changes in alignment.
While trauma, disease, infection, genetics, gout, and neuropathy may lead to secondary osteoarthritis, primary OA is the result of a degeneration that occurs with normal use. This wear and tear of the joint becomes more prevalent with advancing age.
Changes to Cartilage
The progressive loss of cartilage is a process that involves three overlapping stages: cartilage matrix (surroundings) damage, cartilage chondrocyte (cell) response to tissue damage, and decline of chondrocyte synthetic response (ability to maintain its environment)
Changes to Bone
As cartilage degenerates, there is increased exposure of the bone supporting the joint (subchondral bone). With time, the subchondral bone becomes dense (sclerosis) with cyst formation. Cartilage does not regenerate on its own starting about age 40. With time the aborted reparative process may result in osteophyte formation (spurs).
Changes to Periarticular Soft Tissues (in and around the joint)
Synovitis develops (inflammation of the joint lining) because of the release of inflammatory factors by the chondrocytes. A vicious cycle continues with further break down of cartilage followed by thickening of the joint capsule and shortening leading to loss of motion. Muscle undergoes atrophy (shrinkage and weakening) with the relative inactivity of the joint because of pain leading to instability
Changes to Alignment
Abnormal hip-knee-ankle alignment can accelerate structural changes; varus malalignment (bowed leg) increases medial compartment (inner side of the knee) disease fourfold, and valgus (knock knee) malalignment increases lateral (outer) disease twofold. Whether malalignment is associated with development of osteoarthritis or if malalignment is a result of OA is still a subject of debate. However, it has been demonstrated that malalignment can affect more than cartilage because malalignment predis- poses the patient to bone marrow lesions (nonhealing stress fractures).
Treatment of Osteoarthritis
Life style modification, rehabilitation (physical therapy), complementary and alternative therapy, pain relievers, intraarticular injections (cortisone, hyaluronic acid gels), arthroscopic and joint replacement surgery, and now, regenerative intervention.
Regenerative Intervention (an injection, not an incision)
Cellular intervention is what I do. Biologic solutions through cartilage regeneration is the goal of my practice. My stem cell source is the patient’s own bone marrow. Equally important are growth factors; the latter found in bone marrow and in platelets.
To learn more, visit my web site www.sheinkopmd.com
You may watch my webinar www.ilcellulartherapy.com
Then schedule an appointment 312 475 1893
Tags: arthritis, Clinical Trial. Mitchell B. Sheinkop, Interventional Orthopedics, Knee Pain Relief, Orthopedic Care, Osteoarthritis, regenerative medicine

Apr 7, 2017
I have purposely used the terms interventional orthopedics and cellular orthopedics when referring to regenerative medicine to remind my reader that I am an orthopedic surgeon. Later in life, I graduated into my present role as a clinician seeking to assist a patient in postponing, at times avoiding a major surgical procedure for an arthritic or otherwise compromised joint. You will note that I limit my discussion and topic matter to the musculoskeletal system and, do not allow vanity or greed to suggest that I am willing to expand my scope of care directed to conditions and diseases for which I am willing to provide treatment. In my 37-year commitment to reconstructive orthopedics and joint replacement surgery, I did not increase my scope of services outside the musculoskeletal system and I won’t consider anything more in my regenerative medicine undertakings, today.
To take things a bit further, when it comes to cartilage damage in any joint and from any causation, there are three categories of care: Palliative, Reparative and Restorative. In the first category, palliative, I do offer anti-inflammatory prescription, cortisone injection and hyaluronic intervention. At times, for those who meet inclusion criteria, I even enroll patients in an amniotic fluid clinical trial for pain management when deemed appropriate knowing there is no regenerative or even reparative potential therein. Reparative may take place during a Bone Marrow Concentrate procedure; but my goal is Restorative (Regeneration). The only FDA complaint method for delivering stem cells to an arthritic joint is the use of your aspirated and then concentrated bone marrow from your pelvis. In spite of the misleading and false news to be found on the various web sites, in order for stem cells to be separated from fat, an enzymatic digestion must take place and that manipulation renders adipose derived stem cell usage contrary to FDA mandates. Furthermore, there is no published scientific literature demonstrating adipose derived stem cells are of value in the care and treatment of an arthritic or otherwise altered joint function.
When you decide to seek out a provider of regenerative services, a very important part of the decision- making process should be to question that provider as to whether services are limited to the musculoskeletal system and what outcomes and data of that particular practice experiences? I have noted recently that my data and outcomes are being posted on web sites around the country as if the results were being achieved in settings other than mine.
If you want to learn more about postponing or perhaps even avoiding surgery for a joint that alters your quality of life, call 312-475-1893.
To learn more, check out my web site at www.Sheinkopmd.com
View my webinar at www.ilcellulartherapy.com
Tags: arthritis, Bone Marrow Concentrate, Clinical Trial. Mitchell B. Sheinkop, Concentrated Stem Cell Plasma, Interventional Orthopedics, joint pain, joint replacement, Knee, knee pain, regenerative medicine, stem cells

Feb 4, 2017
Options for treating patients with osteoarthritis of their joints are historically limited to pain medication, anti-inflammatory medications, steroids, physical therapy, chiropractic care, or any combination thereof. These treatments provide temporary symptom relieving care, but do not offer therapeutic benefit in altering the degenerative disease progression. While pain medication, steroids and anti-inflammatories may help temporarily with pain management, they do not have a long-lasting impact on healing of articular cartilage in the arthritic joint. Without a regenerative therapy, the osteoarthritis will continue to progress, and ultimately will result in a total joint replacement as the only option to manage pain. While the majority of joint replacements have proven successful, there is an inherent complication risk; sufficiently significant enough that a patient prior to surgery might want to look for a means of postponing, perhaps avoiding a joint replacement. A treatment that might slow or even reverse the degenerative process. Four and a half years into my Cellular Orthopedic initiative, I believe the evidence I have compiled supports the use of the patient’s own concentrated bone marrow derived cells (BMC) in combination with the patient’s own concentrated Platelets and Plasma as an alternative to a major joint replacement.
Accomplished in a surgi-center under local anesthesia, an intra-articular injection, with image confirmation of needle and orthobiologic placement, is performed with Concentrated Bone Marrow mixed with concentrated Platelets and Plasma. Recently, based on publications in the scientific literature, I have added a subchondroplasty, that is an injection of some Bone Marrow Concentrate and Platelet Rich Plasma Concentrate into the bone adjacent to the joint. After six months of having introduced the subchondroplasty when indicated to the intra-articular injection, the presumptive evidence encourages me to continue the combined procedure. I started with the knee and I have extended subchondroplasty to the hip and shoulder.
When I began the combined procedure, that is injecting Bone Marrow Concentrate into the joint as well as into the bone adjacent to the joint, I limited the indication to patients under age 60. In August of 2016, a clinical paper was published reviewing the results of said interventions into patients older than 60; Total Knee Arthroplasty versus cell therapy in bilateral knee osteoarthritis in patients older than 85 years. Space doesn’t allow me to reproduce the entire article but in those patients who had a TKR on one side and a combined intervention into the knee and into the bone supporting the knee with Bone Marrow Concentrate, the majority of patients expressed a preference for the stem cell therapy.
To learn more or schedule an appointment, call (312) 475-1893
You may visit my blog posted on my website www.sheinkopmd.com
You may view my webinar at www.ilcellulartherapy.com
Tags: arthritic pain, arthritis, Bone Marrow Concentrate, Interventional Orthopedics, Osteoarthritis, stem cells


